As virus flares globally, governments use new strategies to target hot spots
Submitted by mike kraft on
Submitted by mike kraft on
Submitted by mike kraft on
A $5 rapid test for the coronavirus may be nearly as effective as the slower, more complex polymerase chain reaction test for identifying people who may spread the coronavirus, a novel experiment has found.
The study, conducted by scientists at the University of California, San Francisco, is among the first head-to-head comparisons of a rapid test and the P.C.R. diagnostic tool under real-world conditions.
But the number of participants was comparatively small, and the data have not been peer-reviewed or published. A rapid test still cannot conclusively determine that an individual is not infected; the tests are intended primarily to detect the presence of high levels of the virus, rather than its absence, and are authorized only to evaluate symptomatic people.
At the moment, anyone who has been exposed to the virus should be tested by P.C.R., said Joseph DeRisi, an infectious disease expert at U.C.S.F. and a co-leader of the project.


Submitted by mike kraft on
 US regulators approve 1st treatment for Ebola U.S. regulators Wednesday approved the first drug for the treatment of Ebola. The Food and Drug Administration OK'd the drug developed by Regeneron Pharmaceuticals for treating adults and... AP NEWS
US regulators approve 1st treatment for Ebola U.S. regulators Wednesday approved the first drug for the treatment of Ebola. The Food and Drug Administration OK'd the drug developed by Regeneron Pharmaceuticals for treating adults and... AP NEWS U.S. regulators Wednesday approved the first drug for the treatment of Ebola.
The Food and Drug Administration OK’d the drug developed by Regeneron Pharmaceuticals for treating adults and children. It was tested during an outbreak in Congo that killed nearly 2,300 people before it ended in June.


Submitted by mike kraft on
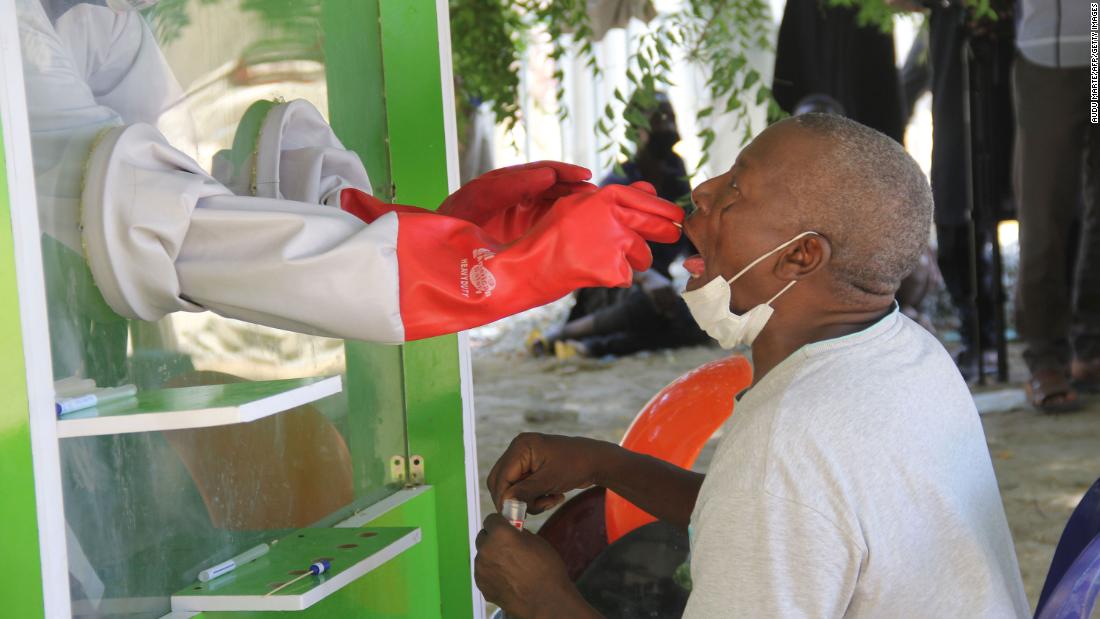 Nigerian scientists develop cheaper and faster Covid-19 test kits Scientists in Nigeria have developed a cheaper and faster Covid-19 test kit that will enable testing to be ramped up in a place that has faced kit shortages and chronic under-testing of a massive population, according to the country's health authorities. CNN
Nigerian scientists develop cheaper and faster Covid-19 test kits Scientists in Nigeria have developed a cheaper and faster Covid-19 test kit that will enable testing to be ramped up in a place that has faced kit shortages and chronic under-testing of a massive population, according to the country's health authorities. CNN Submitted by mike kraft on
 COVID-19 test that produces results in 15 minutes approved in Europe The move comes as Europe is seeing a resurgence of coronavirus cases. TheHill
COVID-19 test that produces results in 15 minutes approved in Europe The move comes as Europe is seeing a resurgence of coronavirus cases. TheHill A rapid coronavirus test that can deliver results in just 15 minutes has been cleared for use in Europe and is expected to hit the market next month.
Submitted by mike kraft on
Submitted by mike kraft on
 Nearly 1M who died of COVID-19 also illuminated treatment The nearly 1 million people around the world who have lost their lives to COVID-19 have left us a gift: Through desperate efforts to save their lives, scientists now better understand how to treat... AP NEWS
Nearly 1M who died of COVID-19 also illuminated treatment The nearly 1 million people around the world who have lost their lives to COVID-19 have left us a gift: Through desperate efforts to save their lives, scientists now better understand how to treat... AP NEWS The nearly 1 million people around the world who have lost their lives to COVID-19 have left us a gift: Through desperate efforts to save their lives, scientists now better understand how to treat and prevent the disease — and millions of others may survive.
Submitted by mike kraft on
 Robots target coronavirus with ultraviolet light at London train station Robots that can kill the coronavirus with ultraviolet light have been brought in at one of London's biggest train stations, St Pancras International, as it tries to restore customer confidence in the safety of travel hubs. U.S.
Robots target coronavirus with ultraviolet light at London train station Robots that can kill the coronavirus with ultraviolet light have been brought in at one of London's biggest train stations, St Pancras International, as it tries to restore customer confidence in the safety of travel hubs. U.S. Submitted by mike kraft on
Submitted by mike kraft on